CONCLUSIONS
MDS are serious life-threatening hematologic malignancies in which a heterogeneous group of clonal disorders results in ineffective hematopoiesis. An unmet need remains for new therapies for patients (pts) with LR-MDS who are red blood cell transfusion dependent (RBC-TD) and R/R to or ineligible for ESAs. Imetelstat, an oligonucleotide, is a first-in-class telomerase inhibitor that targets cells with high telomerase activity by direct binding to the RNA template of telomerase. In the IMerge phase 3 trial, imetelstat produced higher rates of TI for ≥8 weeks, ≥24 weeks, and ≥1 year (39.8%, 28.0%, and 17.8%) than placebo (15.0%, 3.3%, and 1.7%) in pts with non-del(5q) LR-MDS that was RBC-TD, R/R to/ineligible for ESAs, and naïve to lenalidomide or hypomethylating agents (HMAs; Platzbecker et al. EHA 2023. Abstr S165). We report characteristics and clinical benefit for pts with sustained TI for ≥1 year from this trial.
IMerge (MDS3001, NCT02598661) is a global, double-blind, randomized, placebo-controlled, phase 3 trial of imetelstat in RBC-TD, ESA-R/R, non-del 5(q) lenalidomide/HMA-naïve LR-MDS. The primary end point was 8-week TI rate; secondary end points included safety, 24-week TI, duration of response, hematologic improvement, and MDS response. Exploratory end points included assessment of cytogenetic response and mutational status with clinical response. The proportion of pts with>1-year TI and other binary end points, were summarized with percentage and 95% 2-sided exact Clopper-Pearson CI. The Kaplan-Meier method was used to estimate the distribution of TI.
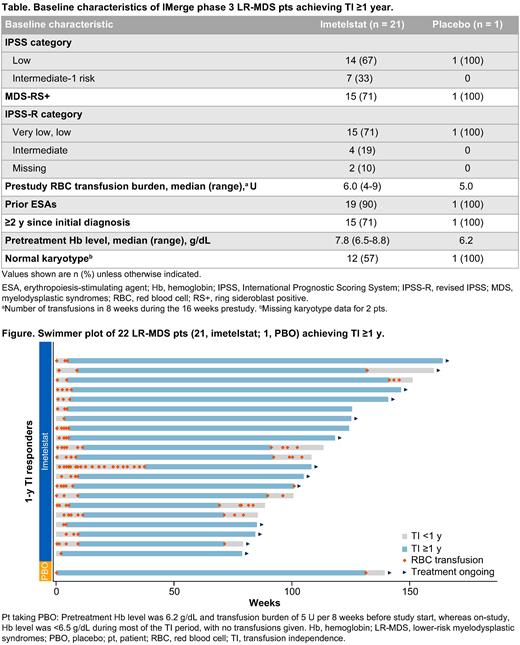
Of 118 pts receiving imetelstat, 21 (17.8%; 95% CI, 11.4-25.9) achieved ≥1-year sustained TI, representing 45% of ≥8-week TI (21 of 47 pts) and 64% of ≥24-week TI (21 of 33 pts); of 60 pts receiving placebo plus supportive care, 1 (1.7%; 95% CI, 0-8.9) achieved ≥1-year TI. Of the ≥1-year TI imetelstat responders, 15/21 (71.4%) had ring sideroblasts, as did the 1 placebo pt. The median prior RBC transfusion burden was 6 U over 8 weeks (range, 4-9 U) for the imetelstat group and 5 U for the placebo pt. Additional baseline characteristics are in the table. Pts received imetelstat for a median of 101.1 weeks (range, 75.1-163.9 weeks) and a median of 24 cycles (range, 18-41 cycles). The median duration of TI for imetelstat ≥1-year TI responders was 123 weeks (95% CI, 80.4 to not evaluable); the median increase in hemoglobin during the longest TI interval was 5.18 g/dL (range, 2.67-13.76 g/dL) for the imetelstat group vs 1.67 g/dL for the placebo pt. After a median follow-up of 125 weeks, none of the patients with ≥1 year TI on either arm progressed to acute myeloid leukemia (AML). Of the pts receiving imetelstat, 7 had an abnormal karyotype at baseline, of which 6 had reduction in the cytogenetic abnormal clones (4 with cytogenetic complete response and 2 with cytogenetic partial response by independent review committee). Mutation data were available for 18 pts receiving imetelstat, all with SF3B1 mutations present at baseline, and multiple of these pts concurrently had TET2, DNMT3A, ASXL1, or JAK2 mutations. The maximal reduction ranged from −6% to −100% in SF3B1 variant allele frequency (VAF) in these pts, and 13 of 18 (72.2%) achieved ≥50% VAF reduction, including 7 with complete elimination of the VAF. Reduction in other concurrent mutations was also observed in these pts. Safety was consistent with that previously reported; most frequent adverse events were reversible grade 3 or 4 thrombocytopenia and neutropenia. At the time of data cutoff (May 10, 2023), 13 pts receiving imetelstat and the pt receiving placebo were ongoing (Figure); of the 8 who discontinued treatment, 7 had loss of response, and 1 was due to adverse event. Analyses for progression-free and overall survival were not evaluable as of this cutoff date (insufficient follow-up).
Treatment with imetelstat resulted in ≥1-year sustained, continuous TI in 17.8% of pts in the IMerge phase 3 trial. In this ESA-R/R/ineligible population with a high prior transfusion burden, a reduction to 0 RBC transfusions for ≥1 year represents an opportunity to achieve relief from iron overload and other transfusion associated complications, and decreased demand on already limited blood product supply. Furthermore, durable TI and meaningful reductions in mutational burden suggest imetelstat may have disease-modifying activity.
OffLabel Disclosure:
Platzbecker:Silence Therapeutics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Fibrogen: Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Syros: Consultancy, Honoraria, Research Funding; Janssen Biotech: Consultancy, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Curis: Consultancy, Research Funding; Celgene: Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Amgen: Consultancy, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Geron: Consultancy, Research Funding; AbbVie: Consultancy; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding. Komrokji:AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy. Zeidan:Gilead: Consultancy, Honoraria; Astex: Research Funding; ALX Oncology: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Shattuck Labs: Research Funding; Pfizer: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Syros: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Fenaux:French MDS Group: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Sekeres:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kurome: Consultancy, Current holder of stock options in a privately-held company; Geron: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Savona:Incyte: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; AbbVie: Consultancy; BMS/Celgene: Consultancy; Forma: Consultancy; Geron: Consultancy; Karyopharm: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy; Ryvu: Consultancy, Current equity holder in publicly-traded company; Sierra Oncology: Consultancy; Taiho: Consultancy; Takeda: Consultancy; TG Therapeutics: Consultancy; Astex: Research Funding; ALX Oncology: Research Funding. Madanat:GERON: Consultancy; Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement; Rigel Pharmaceuticals: Honoraria; Sierra Oncology: Honoraria; Morphosys: Honoraria, Other: travel reimbursement; Stemline therapeutics: Honoraria; Taiho oncology: Honoraria; Novartis: Honoraria; OncLive: Honoraria; MD Education: Honoraria. Jonášová:AbbVie, BMS/Celgene, Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie, BMS/Celgene: Other: travel, accommodations, and expenses . Illmer:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sherman:Geron: Current Employment, Current equity holder in publicly-traded company. Berry:Geron: Current Employment, Current equity holder in publicly-traded company. Riggs:Geron: Current Employment, Current equity holder in publicly-traded company. Xia:Geron: Current Employment, Current equity holder in publicly-traded company. Navada:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Wan:Geron: Current Employment, Current equity holder in publicly-traded company. Huang:Geron: Current Employment, Current equity holder in publicly-traded company. Feller:Geron: Current Employment, Current equity holder in publicly-traded company. Santini:Syros: Other: Advisory boards; Servier: Other: Advisory boards; Novartis: Other: Advisory boards; Otsuka: Other: Advisory boards; BMS/Celgene: Other: Advisory boards; Gilead: Other: Advisory boards; Geron: Other: Advisory boards; CTI: Other: Advisory boards; Janssen: Other: travel grant; AbbVie: Other: Advisory boards.
Imetelstat to treat lower-risk myelodysplastic syndromes relapsed/refractory to or ineligible for erythropoiesis-stimulating agents

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal